

Vol. 38 (Nº 44) Año 2017. Pág. 12
Tamires B. OLIVEIRA; Karollyne R. S. SILVA; Dielle T. F. TEIXEIRA; Milena P. MORAES; Andresa S. COSTA; Bruno M. MALTAROLO; Ellen G. S. LIMA; Glauco A. S. NOGUEIRA 1; Cândido F. O. NETO
Recibido: 09/05/2017 • Aprobado: 02/06/2017
ABSTRACT: The aim of this work was to evaluate the biometry and carbon metabolism in young plants of Visgueiro submitted to drought. The experiment was conducted in a greenhouse belongs to Federal Rural University of Amazon (UFRA), in Belém - Brazil. Were analised the relative water content, content of total soluble carbohydrates, sucrose, and the growth variables. There were significant changes in function of the established condition of water stress, directly interfering in its growth and development. |
RESUMO: O objetivo desse trabalho foi avaliar a biometria e o metabolismo do carbono em plantas jovens de Visgueiro submetidas à deficiência hídrica. O experimento foi conduzido em casa de vegetação da Universidade Federal Rural da Amazônia (UFRA), campus Belém, Pará. Foram analisados o conteúdo relativo de água, as concentrações de carboidratos solúveis totais, sacarose assim como, as variáveis de crescimento, houveram mudanças significativas em função da condição estabelecida de estresse hídrico, interferindo diretamente em seu crescimento e desenvolvimento. |
The Amazonian forest species Parkia pendula (Willd.) Benth. Ex Walp, popularly known as Visgueiro, has been studied for presenting important characteristics in the economic and environmental scope. Its wood is of low density, this favors its rational exploration in the timber industry, it has been indicated for the recovery of degraded areas due to its rapid growth and low mortality (PINEDO E FERRAZ, 2008).
However, in order to be successful in these areas it is necessary to deepen studies related to their ability to tolerate water stresses, aiming to know their physiological and biochemical characteristics.
According to Nascimento et al., (2011), water availability is one of the main factors that regulates seedling growth and survival. According to Silva et al. (2009), plants grown in environments with water restriction develop adaptations to tolerate drought. And one of these changes occurs in the reduction of the photosynthetic rate associated with stomatal closure due to changes in leaf water status.
The relative water content (RWC) is an evaluator of the degree of water deficiency of a plant, a variable that is measured in the leaves and allows to quantify to the water in the leaf tissue at a given instant, comparing with the maximum amount of water that it can retain (NASCIMENTO et al., 2011). Water stress is generally perceived as a decrease in plant growth and is associated with changes in carbon and nitrogen metabolism (GONÇALVES et al., 2010).
Changes in carbonaceous structures have been related to the breakdown of compounds such as the main sugar starch undergoing hydrolysis and this leads to the accumulation of other soluble sugars such as sucrose. This mechanism is developed by hydrolytic enzymes that participate directly in the osmotic adjustment (MORANDO et al., 2014).
These carbohydrates participate in numerous metabolic processes such as photosynthesis, respiration, fixing of CO2, (COSTA, 2012), and under drought, Part of the carbohydrates arranged in the cytoplasm, are used in the activation of the mechanisms of tolerance to the drought, independent of the mechanism in question LISAR et al., (2012), looking for osmotic adjustment (LIU et al., 2011).
Other responses in plants under conditions of water stress are related to biomass production, water stress reduces growth, number of leaves, increases senescence and leaf fall, and restricts growth and stem diameter, in contrast increases and accelerates the development of the root system. The plants tend to invest more in biomass in the root system, allowing greater root growth and, consequently, an increase in nutrient absorption capacity (DUARTE, 2012).
Therefore, based on the above, this work had as objective to evaluate the carbon metabolism and biometry of young plants of Visgueiro submitted to water deficit.
The conduction of the experimente was realized in a greenhouse and the physiological analyzes carried out in the Laboratory of Biodiversity Studies in Upper Plants (EBPS), both belonging to Instituto de Ciências Agrárias (ICA) of Federal Rural University of Amazon (UFRA), campus Belém/Pará.
Were used seedlings of visgueiro (Parkia pendula (Willd.) Benth. ex Walp), Originating from the AIMEX company, with approximately seven months of age with 10-15 cm of height, being conditioned in plastic pots with capacity for 7.5L. Having as substratum black clay earth. The seedlings were acclimatized in a shaded environment (50%) and irrigated daily to keep them in the field capacity. They received 5 mL of macro and micronutrients every 30 days until the beginning of the experiment, in the form of Arnon nutrient solution, modified in the Laboratory of Biodiversity in Higher Plants (EBPS), UFRA. The plants were submitted to two water regimes: irrigated (control) and water deficit, in which the imposition of the water deficit was obtained by the suspension of the irrigation in the period of 10 days, being the time 0 (zero days of water deficit), time 1 (5 days of water deficiency) and time 2 (10 days of water deficiency), with 5 replications, totaling 30 experimental units, each unit being composed of one plant / pot.
Plant samples were collected at 5:00 am in the greenhouse, and the discs were removed from the fully expanded primary leaves of each of the replicates to determine the relative water content (RWC). Biometric measurements were then made. Afterwards, the plants were separated into leaves and roots, and placed in a forced air oven at 70 ° C for milling until the fine powder was obtained for analysis of the carbohydrate and sucrose determination, being stored in falcon tubes.
The RWC was determinated according to the method described by Slavick (1979), with some modifications. The results were expressed as a percentage, for better interpretation of the data.
Total soluble carbohydrates (CST) were determined according to the colorimetric method described by Dubois et al., (1956), modified as follows: Vegetable samples were homogenized in 5 mL of distilled water and the resulting homogenate incubated at 100 ° C for 30 Minutes. After centrifugation at 700 g for 10 minutes, the supernatant was collected and the extraction procedure repeated twice. The supernatants were combined and homogenized and, from the resulting final extract, an aliquot of 20 μL was sampled for the remaining steps. To each aliquot was added 480 μL of deionized water and, after stirring for 15 minutes, 500 μL of 5% phenol and 2.5 mL of concentrated sulfuric acid were added to each sample. After vigorous shaking for 20 minutes, the reading was performed by a spectrophotometer (GenesysTM10 series, Thermo Electron Co, Wisconsin, USA) at 490 nm.
For determination of sucrose the method of Van Handel (1968), has undergone some modifications. The samples were macerated in 1.5 ml of MCW (12: 5: 3, v / v / v methanol: chloroform: water) and stirred for 20 minutes. The homogenate was centrifuged at 500 g for 30 minutes at room temperature. After collection of the supernatant, the extraction procedure was repeated twice consecutively and the supernatants pooled and their final volume determined. After stirring, heating at 100 ° C for 10 minutes and cooling in an ice bath, a volume of 3.0 ml of 0.2% antrone solution (in sulfuric acid) was added to each tube. The mixture was stirred and heated again at 40 ° C for 20 minutes and, after cooling, the reading was carried out at 620 nm by means of a spectrophotometer (GenesysTM10 series, Thermo Electron Co., Wisconsin, USA).
The experimental design was completely randomized in a 2 x 3 factorial scheme (two water conditions: control and water deficit, and three evaluation times), with 5 replications, totaling 30 experimental units.
The experimental results were submitted to analysis of variance (ANOVA), and when significant differences were verified the averages were compared by the Tukey test at the 5% level of significance. Regression analysis was performed on the variables, whose significance was verified by the F-test (P <0.05). Statistical analyzes were performed using the Assistat program 7,7.
The relative content of water in leaf tissues of plants submitted to water deficiency decreased significantly over time when compared to control plants (Figure 1). The irrigated plants maintained averages of 76% throughout the experiment. The values presented by plants submitted to water deficiency in the periods 0, 5 and 10 days were 73.5%, 62.47% and52.99% respectively.
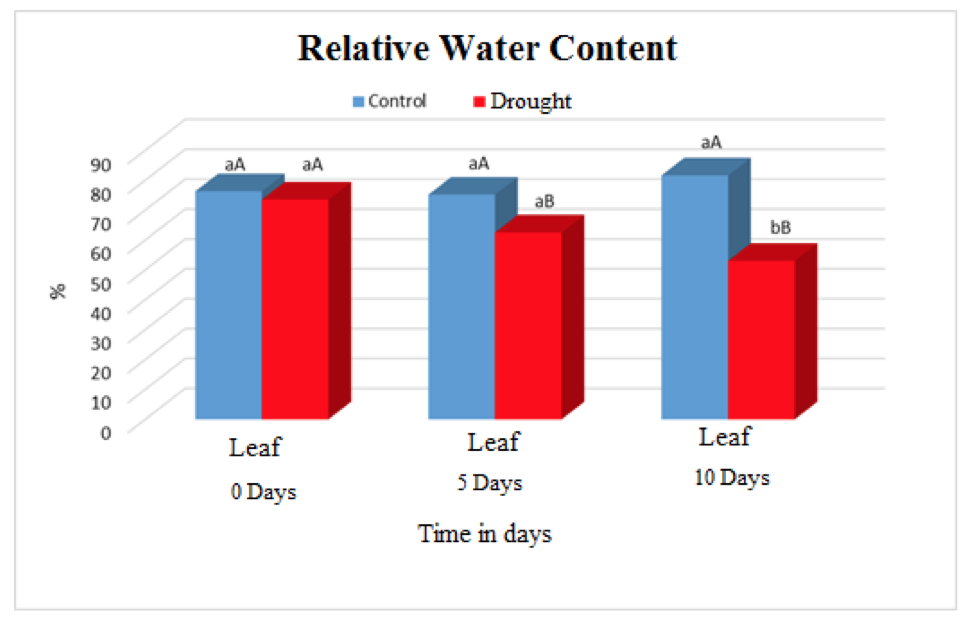
Figure 1. Relative water content in leaves of Visgueiro (Parkia pendula (Willd.) Benth. ex Walp) submitted to 0, 5 and 10 days of drought. Means followed by the same uppercase or lowercase letter do not differ from one another by the tukey test at 5% probability. The lowercase letters compare the values between the treatments and the upper case letters compare the values between the water regimes.
This decrease in CRA of the weed plants can be explained in part by the retention of water molecules in the soil colloids, preventing their displacement and consequently directly interfering in the formation of the concentration gradient and water balance of these plants according to (COOPER et al. 2012).
The reduction of water caused an increase in tension in the xylem vessels, causing the plants to perform a necessary force to absorb the water and to be transported to the aerial part (MOLLE, 2011). As well as restricting the hydraulic conductivity of the roots, inhibiting the metabolic activities and the reduction in the ATP production that ends up limiting the energy supply for root growth, causing a reduction in the development and physiological processes of the plant (COSTA, 2012).
However, the plants adjusted osmotically by increasing the concentrations of sugars according to (Figure 2A and 2B), which provided an increase in energy for the uptake of water in the soil colloids and to continue with their metabolic processes.
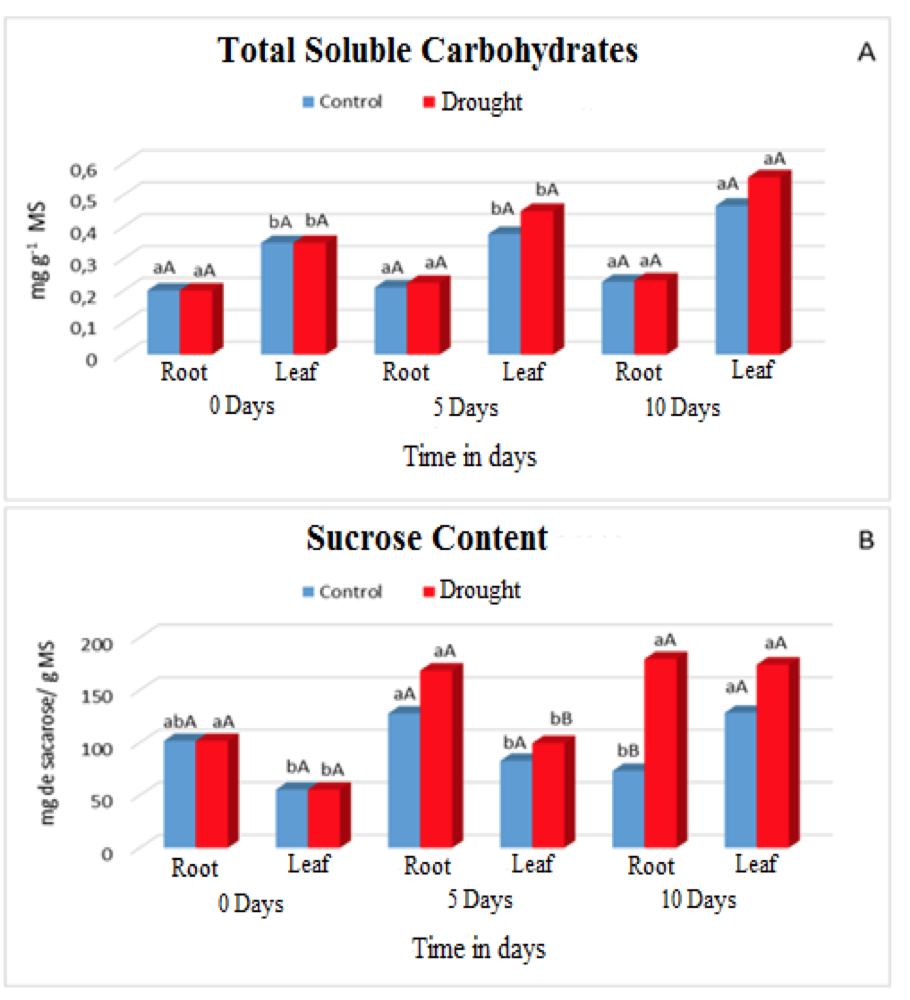
Figure 2. Contents of Total soluble carbohydrates (A) and Sucrose (B) in leaves and roots of Visgueiro plants (Parkia pendula (Willd.) Benth. ex Walp) submitted to 0, 5 and 10 days of drought. Means followed by the same uppercase or lowercase letter do not differ from one another by the tukey test at 5% probability. The lowercase letters compare the values between the treatments and the upper case letters compare the values between the water regimes.
In the plants submitted to water deficiency the concentrations of total soluble carbohydrates and sucrose increased both in the leaves and in the roots (Figure 2A and 2B), for the carbohydrate concentrations there were statistical differences only in the area and in the 10th day of evaluation when compared with Control plants. For the concentrations of sucrose as the main sugar in the plants, the highest amounts accumulated in the roots, with statistical difference between the treatments on the 10th day of evaluation.
The synthesis of these compounds is due to a defense mechanism called osmoregulation, which plants exhibit when they are submitted under adverse conditions such as lack of water, this provides an increase in energy gain, thereby avoiding their cellular dehydration and proportionally the maintenance of their functions Metabolic diseases.
This defense system causes degradation of non-structural carbonates such as starch, which are compartmentalized in chloroplastídicos, by the enzymes amylases, that act mainly in these conditions of stress. After the degradation occurs, the accumulation of these compounds in the vacuole or cytosol of root cells contributes to the maintenance of water balance and the preservation of the integrity of proteins, enzymes and cell membranes (ASHRAF et al., 2011). In the case of sucrose it undergoes hydrolysis, consequently releasing the hexoses, this action is intermediated by enzymes specific to SuSy and invertases that regulate the entry of sucrose in different directions of use.
The initial phase of plant growth is extremely dependent on water, given that without this solvent the biometric variables are changed greatly. And this was observed in this study where the variables height, number of leaflets, leaf number and root length were affected in relation to their growth (Figure 3A, 3C, 3D and 3E), for the variable in diameter was observed a Constant until the end of the experiment (Figure 3B).
The variable height of the plants under water stress showed a much smaller growth when compared to the control plants, this corresponded around 20%. This was due to the lack of water which reduces turgor pressure and, consequently, sap flow through conducting vessels, a fact that tends to decrease cell elongation and plant growth and development.
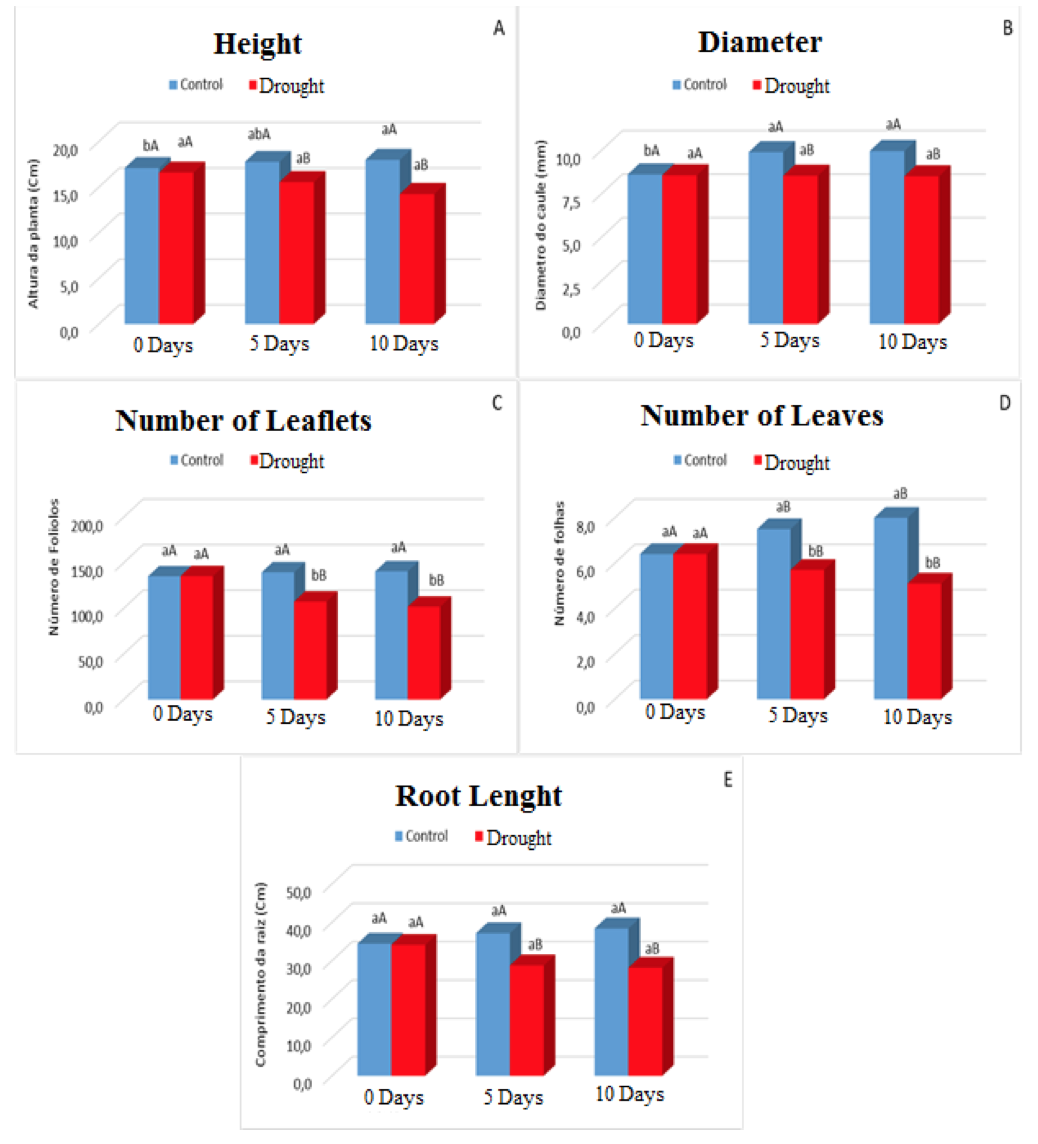
Figure 3. Height (A), Stem diameter (B), Number of Leaflets (C), Number of Leaves (D) and Root Lenght (E) in plants of Visgueiro (Parkia pendula (Willd.) Benth. ex Walp) submitted to 0, 5 and 10 days of drought. Means followed by the same uppercase or lowercase letter do not differ from one another by the tukey test at 5% probability. The lowercase letters compare the values between the treatments and the upper case letters compare the values between the water regimes.
For growth in diameter, dependent on exchange activities, the stimulation for development occurs from carbohydrates produced by photosynthesis and translocated hormones from the apical regions. More when the stomata are closed, possibly interferes with the size of the stem diameter, because they negatively influence the production of carbonate assimilates, increasing the activity of oxidizing enzymes that results from the elevation of the temperature of the plant that causes the consumption of photoassimilates to occur and then reduction of stem growth (VIEIRA et al., 2011).
The stress condition affected the development of leaves and leaflets (Fig. 3C and 3D), where this behavior could be interpreted as a line of defense to water loss (PAIVA; OLIVEIRA, 2006). According to Nascimento et al., 2011 the water limitation does not only affect the size of the leaf area, but also the amount of leaves that the plants have due to reducing the emission and growth of the branches, where such reduction according to Dantas, 2014, is associated with the reflex Of the reduction of the divisions of the cell expansion, caused by the lower availability of water, adapting morphological to the drought, since the reduction of these also diminishes the leaf surface subject to perspiration.
Soils with water deficiency the plants tend to present greater root growth and a lower growth of the aerial part, because when water is lacking in the superficial layers of the ground the plant invests in the root growth so that greater contact of the roots occurs with the water of the deeper layers. For Scalon et al., 2011, the continuity of root growth, under conditions of lower water availability, depends on the maintenance of a minimum turgor pressure in the cells, which is considerable to allow the cellulosic wall elongation and cell growth. When the water potential is reduced in the roots, a rapid osmotic adjustment is observed, helping to restore the turgor pressure and allowing the maintenance of cell elongation (HSIAO; XU, 2000).
The water stress condition established for a period of 10 days was sufficient to alter the metabolism of the plants of Parkia pendula (Willd.) Benth. Ex Walp. The photosynthetic capacity was altered greatly, interfering directly in the carbon metabolism of these plants, resulting in changes in their growth. However, it can be observed that the species under study, even showing a reduction in its metabolism and growth, did not die, trying biochemical and physiological adjustments to overcome the water stress. Due to this, the advances in research on stress caused by the absence of water in visgueiro plants should be deepened and valid for new responses in the biochemical and physiological field.
ASHRAF, M.; AKRAM, N. A; ALQURAINY, F.; FOOLAD, M. R. (2011) Drought tolerance: roles of organic osmolytes, growth regulators, and mineral nutrients. Advances in Agronomy, v.111.
COOPER, M.; ROSA, D. J.; MEDEIROS, J. C.; OLIVEIRA, T. C. de; TOMA, R. S., JUHÁSZ, C. E. D. (2012) Hydro-physical characterization of soils under tropical semi deciduous forest. Scientia Agricola, v. 69, n. 2.
COSTA, M. F. (2012) Dicionário de Termos Médicos. Editora: Porto Editora, Coleção: DICIONÁRIOS TEMÁTICOS. p. 1584.
DUARTE, A.L.M. (2012) Efeito da água sobre o crescimento e o valor nutritivo das plantas forrageiras. Pesquisa & Tecnologia, vol. 9, n. 2.
DANTAS, S. G. (2014) Crescimento inicial e morfologia foliar em plantas de Enterolobium contortisliquum (Vell.) Morong. E Erythrina velutina Mart. Ex Benth, sob estresse hídrico. Dissertação (Mestrado em Ciências Florestais) – Universidade Federal de Rio Grande do Norte, Macaíba.
DUBOIS, M.; GILLES, K. A.; HAMILTON, J. K.; REBERS, P. A.; SMITH, F. (1956) Colorimetric method for deterination of sugars and related substances. Analytical Chemistry, v. 28.
GONÇALVES, E.R.; FERREIRA, V.M.; SILVA, J.V.; ENDRES, L.; BARBOSA, T.P.; DUARTE, W.G. (2010) Trocas gasosas e fluorescência da clorofila a em variedades de cana-de-açúcar submetidas à deficiência hídrica. Revista Brasileira Engenharia Agrícola e Ambiental, v.14, n.4.
HSIAO, T.C.; XU, L.K. (2000) Sensitivity of growth of roots versus leaves to water stress: biophysical analysis and relation to water transport. Journal of Experimental Botany, Oxford, v. 51.
LISAR, S. Y. S.; MOTAFAKKERAZAD, R.; HOSSAIN, M. M., RAHMAN, I. M M. (2012) Water stress in plants: Causes, effects and responses. In: Water Stress, Edited by: Ismail M. M. Rahman and Hiroshi Hasegawa. 1–14 Rijeka, Croatia: In Tech.
LIU, C.; LIU, Y.; GUO, K.; FAN, D.; LI, G.; ZHENG, Y.; YU, L.; YANG, R. (2011) Effect of drought on pigments, osmotic adjustment and antioxidant enzymes in six woody plant species in karst habitats of southwestern China, Environmental and Experimental Botany, v. 71.
MOLLE, F. R. D. Alterações no metabolismo de xiloglucano de reserva em plântulas de Hymanaea courbaril L. (Hayne) Lee & Lang. submetidas ao déficit hídrico. (2011). Tese (Doutorado biodiversidade vegetal e meio ambiente) - Instituto de Botânica da Secretaria de Estado do Meio Ambiente, São Paulo.
MORANDO, R.; SILVA, A.O.; CARVALHO, L.C.; PINHEIRO, M.P.MA. DÉFICIT HÍDRICO: EFEITO SOBRE A CULTURA DA SOJA. (2014) Journal of Agronomic Sciences, Umuarama, v.3, n. especial.
NASCIMENTO, H. H. C. do; NOGUEIRA, R. J. M. C.; SILVA, E. C.; SILVA, M. A. (2011) Análise do crescimento de mudas de jatobá (Hymenaea courbaril L.) em diferentes níveis de água no solo. Revista Árvore, v.35, n.3, Edição Especial, p.617- 626.
PAIVA, R.; OLIVEIRA, L. M. (2006) Fisiologia e Produção Vegetal. 1ª Ed. Lavras-MG: Editora UFLA.
PINEDO, G.J.V.; FERRAZ, I.D.K. (2008) Hidrocondicionamento de Parkia pendula (Benth ex Walp): sementes com dormência física de árvore da Amazônia. Revista Árvore, Viçosa-MG, v.32, n. 1.
SCALON, S. P. Q.; MUSSURY, R. M.; EUZÉBIO, V. L. M.;KODAMA, F. M.; KISSMANN, C. (2011) Estresse hídrico no metabolismo e crescimento inicial de mudas de mutambo (Guazuma ulmifolia Lam.). Ciência Florestal, Santa Maria, v. 21, n. 4.
SILVA, J. R. R. E. (2009) Comportamento Ecofisiológico de Plantas Jovens de Andiroba (Carapa guianensis Aubl.) Sob Dois Regimes Hídricos. Dissertação (Mestrado em Agronomia). Universidade Federal Rural da Amazônia – UFRA, Belém-PA.
SLAVICK, B. (1979) Methods of studying plant water relations. New York, Springer Verlang. p. 449.
VAN HANDEL, E.(1968)Direct micro determination of sucrose. Analytical Biochemistry, v. 22, p. 280-283.
VIEIRA, E. A.; GOMES, A. S. (2011) Desenvolvimento inicial de plantas de pau-terra-do-cerrado sob diferentes regimes hídricos. Evolução e Conservação da Biodiversidade, v. 2 (1).
1. Engenheiro do (Departamento em ciências agrárias) da Universidade Federal Rural da Amazônia, Pará, Brazil. Email: glauand@yahoo.com.br