

Vol. 38 (Nº 22) Año 2017. Pág. 12
Marcia Regina EWALD 1; Daniela da Gama e Silva Volpe Moreira de MORAES 2
Recibido: 15/11/16 • Aprobado: 02/16/2016
2. Hazardous substances management system (HSMS)
3. HSMS: Case study in Brazilian electromedical sector
4. Other trends in establishing a HSMS
ABSTRACT: The electronic industry in Brazil is increasingly involved with environmental performance issues, including those ones related to restriction of substances hazardous to human health and to the environment. The current challenge is to ensure that a specific electronic product does not contain hazardous substances or otherwise that those substances are effectively controlled in all product´s constituent materials and during all stages of the production process. The article aims to present main opportunities and challenges of the Brazilian electronic industry in the implementation of a hazardous substances management system (HSMS), based on ABNT IECQ QC 080000:2010, considering that such implementation is an important tool to confront the challenge of providing environmentally friendly electronic products. |
RESUMO: A indústria de produtos eletroeletrônicos no Brasil está cada vez mais envolvida com as questões de desempenho ambiental, incluindo a necessidade do atendimento a regulamentos que restringem a presença de substâncias que são perigosas para saúde humana e para o meio ambiente. O desafio atual está relacionado com a garantia de que o produto não possui substâncias perigosas ou que as mesmas são controladas de forma efetiva em todos os materiais constituintes do produto e durante todas as etapas do processo produtivo. O artigo tem como objetivo apresentar as principais oportunidades e desafios do setor de eletroeletrônicos brasileiro na implementação de um sistema de gestão de substâncias perigosas (SGSP), baseado na norma ABNT IECQ QC 080000:2010, considerando que esta implementação é uma importante ferramenta no enfrentamento do desafio de oferecer produtos eletroeletrônicos ambientalmente corretos. |
The Brazilian electronic industry is increasingly involved with issues of environmental performance, including the need to meet environmental requirements that imply deep changes or adaptations in production processes.
Furthermore, the incorrect disposal of electronic waste generates serious environmental problems, not only by volume and time they take to decompose, but also by the presence of hazardous substances in its composition, highly detrimental to human health and environment.
Brazil generates approximately 680 thousand tons of electronic waste a year and it is expected to generate 22 million tons of electronic waste between 2001 and 2030 (ROCHA et. al., 2009).
The concern about sustainable production and reduction of hazardous substances in electronic equipments have been the main motivation for the development of regulations that aim to promote a proper disposal after end-of-life treatment and also to reduce the content of hazardous substances in such equipments.
In European Union, discussions about the problem culminated in 2003 in the Waste Electrical and Electronic Equipment Directive (WEEE Directive) (DIRECTIVE 2002/96/EC, 2003) and Restriction of the Use of Certain Hazardous Substances in Electrical and Electronic Equipment Directive (RoHS Directive) (DIRECTIVE 2002/95/EC, 2003). The purpose of WEEE Directive is to contribute to sustainable production and consumption by incentivizing the prevention of WEEE and, in addition, their reduction through reuse, recycling and other forms of recovery of such waste. It also seeks to improve the environmental performance of all operators involved in the life cycle of electrical and electronic equipment (EEE), e.g. producers, distributors and consumers and, in particular, operators directly involved in the collection and treatment of WEEE (DIRECTIVE 2012/19/EU, 2012).
RoHS Directive establishes restriction on the use of hazardous substances in EEE. Restricted substances and maximum concentration values tolerated by weight in homogenous materials are lead; mercury; hexavalent chromium; polybrominated biphenyls (PBB); polybrominated diphenyl ethers (PBDE) (0.1%) and cadmium (0.01%) (DIRECTIVE 2011/65/EU, 2011).
RoHS Directive was the basis for other global regulations on the restriction of hazardous substances including other regulatory actions in countries like China, California, Mexico, Australia, Korea and Japan.
Although Brazil has no specific regulation on the restriction of hazardous substances in EEE, Brazilian Normative Instruction Nº 01/2010 (BRASIL, 2010a), related to environmental sustainability criteria in the procurement of goods, services or public constructions, includes RoHS Directive (DIRECTIVE 2011/65/EU, 2011) as a requirement to be considered in technical specifications for government procurement. Besides the actions linked to the government, private companies are including in their purchasing requirements, in addition to technical requirements of the products, restriction of hazardous substances in EEE.
It is important to note that the restriction of use of hazardous substances in EEE increases possibilities for the recycling of WEEE, their economic profitability also reduces the negative impact on the workers health in recycling facilities. In addition, National Policy on Solid Waste (NPSW), Law Nº 12.305/2010 (BRASIL, 2010b), regulated by Decree Nº 7.404/2010 (BRASIL, 2010c), establishes general guidelines for the entire Brazilian territory, concerning the solid waste management, including WEEE. This Policy brings together the following objectives: encouragement of the adoption of sustainable production and consumption of goods and services; adoption, development and improvement of clean technologies in order to minimize environmental impacts; reduction of the volume and hazardous substances in waste and incentivize environmental labeling.
Due to this scenario, the challenge is to ensure that EEE does not have hazardous substances or even that any possibility of contamination is effectively under control in all constituent materials of the product and during all stages of the production process. Thus, the implementation of an industrial management that emphasizes the control of hazardous substances in a systemic approach is an effective tool to ensure that processes and products are free from hazardous substances. Such guarantee involves the knowledge of customer needs, assessment and monitoring of the supply chain and the entire production process.
The article aims to present main opportunities and challenges in the Brazilian electronic sector to implement a hazardous substances management system (HSMS), considering this implementation important to face the challenge of providing environmentally friendly electronic products.
Facing the need to meet the growing demand for environmentally friendly products, Brazilian companies have started to seek sustainable production processes and new production management tools. In this way, fulfillment of environmental laws or regulations on the restriction of the use of hazardous substances becomes mandatory for manufacturing global approach products.
The RoHS Regulations use self-declaration as the basis of the compliance regime (UK, 2014). In the self-declaration, data about the EEE, its manufacturer and/or the body responsible for marketing are informed.
Despite the apparent simplicity that a self-declaration can denote, the confirmation of shipment of contaminated products to a customer that requires restriction of hazardous substances will result in penalties ranging from products apprehension and huge fines payment to the arrest of those responsible for production of these contaminated electronics.
Therefore, the main concern involving EEE manufacturers is how to ensure that products do not have hazardous substances above the permitted concentrations.
To answer this question, we can examine all possible ways to obtain guarantee of compliance with the requirements, including:
a. Obtain guarantee from suppliers that any restricted substance is present in the product and require maintenance of a permanent record of this guarantee. In this case, there is no warranty that the products aren´t contaminated during the manufacturing process.
b. Conduct tests for determination of hazardous substances in final products to verify supplier’s statements. These tests are costly and destructive, making this option unfeasible.
c. Join to a network about supplier’s compliance to the regulations. This is a limited action and may not cover all the needs of manufacturers.
d. Adopt management system that involves the entire production chain. In this case, the process approach can solve the disadvantages mentioned in the previous sections.
Examining the above options, has been identified the need to establish a management system approach. This management system could serve as a basis for a certification and could drive manufacturers in structuring activities of design for environment and based in clean production tools to achieve the compliance with the requirements of RoHS Directive or other regulatory documents that deal with the restriction of hazardous substances in EEE.
For this purpose, International Electrotechnical Commission Quality Assessment System for Electronic Components developed in 2005 the IECQ QC 080000 Electrical and Electronic Components and Products - Hazardous Substance Process Management System Requirements (HSPM) (IECQ QC 080000, 2005). This document was published in Brazil in 2010, with the title ABNT IECQ / QC 080000:2010 Management System for Hazardous Substances in Products and Electrical and Electronic Components – Requirements (ABNT, 2010).
The specification ABNT IECQ/QC 080000 (ABNT, 2010) is a certifiable management system that provides the minimum requirements for management of hazardous substances, with focus on their control and identification and in establishing plans for their removal.
This specification consists of requirements that are additional to the standard ISO 9001 (ABNT, 2008) and aims to provide subsidies for companies to adapt their processes and products to free of hazardous substances requirements.
The Table 1 shows the possible ways to obtain products free of hazardous substances and exemplifies how HSMS addresses these possibilities in compliance requirements.
Possible ways to obtain assurance that EEE are free of hazardous substances |
Compliance within the scope of HSMS |
Obtain guarantee from suppliers |
Defines the requirements for establishing processes to identify and control the introduction of hazardous substances in products. |
Conduct analysis for determination of hazardous substances in products |
Defines the requirements:
|
Join an information exchange network |
Processes documented and made available to customers, organization and quality management system. |
Table 1- Compliance within the scope of HSMS of the possible ways to obtain
assurance that products are free of hazardous substances
Source: EWALD, 2011.
The main purpose of this specification is to identify, control, measure and record the hazardous substances acquired, manufactured and supplied by companies. In order to achieve this purpose, it is necessary that the whole supply chain be involved in this issue, i.e., suppliers need to restrict the distribution of hazardous substances to electronic producers, which in turn, place on the market products free of hazardous substances, which contribute considerably to minimize environmental impacts.
The management system based on ABNT IECQ/QC 080000 (ABNT, 2010) is applicable to manufacturers, suppliers, customers, and users who want to know the status of the products on the presence of hazardous substances and also want to understand how this situation is determined.
Is possible to note that this management system shows strategy for design and production of environmentally friendly products, comply with legal requirements and customer needs. It also includes guidelines for the management of hazardous substances and requirements for the establishment of procedures for identification and control of any inputs of hazardous substances in products.
It is also important to understand why this management system is in alignment with the requirements of ISO 9001 (ABNT, 2008).
Focusing on the customer and process approach, the compliance with the requirements for restricted substances does not have focus on compliance with European Directives, but also in meeting any requirement for restriction of hazardous substances.
All other requirements of ISO 9001 (ABNT, 2008) are fully applicable in the management of restricted substances with some highlights that will be further discussed.
The complementary approaches to these documents become extremely important and help implementers, because it provides the possibility to incorporate techniques and procedures already established in industries that have the quality management system (ABNT, 2008) implemented.
In this way, it is possible to provide guarantee of compliance with environmental requirements of the product in a consistent basis and with systematic approach, without incurring excessive costs related of new concepts or tools that could become barriers to implementation.
The main subjects and key points that compose the specification ABNT IECQ QC 080000 (ABNT, 2010) are the following:
a. The requirements of ISO 9001 (ABNT, 2008) must be applied with the additional requirements described in ABNT IECQ QC 080000 (ABNT, 2010), however, exclusions are not permitted.
b. The section related to management responsibility (section 5) defines that customer requirements must be determined and fulfilled. It also establishes a policy for hazardous substances and measurable objectives, consistent with this policy, including a timeline for the management of hazardous substances and the preparation of a management plan.
c. For section 6 (related to resource management), in addition to the maintenance of the infrastructure needed to meet customer requirements, it is also emphasized training activities for the identification, use and elimination of hazardous substances in conformity with the management plan.
d. The section 7, related to product realization, has important requirements that cover all stages of the product, from concept to dispatch.
The concept of due diligence is widely used during methodical analysis of information and documents, in order to measure actual and potential risks. Thus, all activities related to section 7 should be systematically planned, documented, checked and recorded, in order to clearly demonstrate proactive actions.
In section 7 is also included a plan where goals are set, processes are established and documented, and resources necessary for the fulfillment of the requirements are defined. Besides, this section includes the determination of customer requirements, as well as, statutory and regulatory requirements.
It must be considered the final destination of the product, regardless the location of the buyer, e.g. a buyer in California who will use the product on a branch in the EU must have the requirements of both destinations fulfilled.
Therefore, the technical knowledge of the product and production process is essential, because producer must have the knowledge about the ability to meet customer requirements, even before confirming acceptance of a supply order.
The section related to design and development (section 7.3) requires that a plan for the identification and use of hazardous substances must be developed, as well as, a plan for the control and elimination of these substances. Thus, even if a product is not designed by a manufacturer, an analysis of the product must be done to define the input data, according to customer specification, project planning and verification, in relation to the input data. All activity must be documented and their records must be kept. For this reason, it is not possible to exclude the section related to projects in this management system, in a different way in a quality management system (ABNT, 2008) it is possible.
The section related to purchasing of products (section 7.4) requires special attention. The producer must select suppliers capable to provide materials and parts free of hazardous substances, after appropriate definition of purchasing specification and preparing the supplier for understanding these requirements. Moreover, it must be established a documented procedure for the inspection and identification of hazardous substances included in purchased goods and systematic for ensuring the traceability of the purchased products.
The process control activity must be applied to actions of product approval, dispatch and post-delivery activities, ensuring that the requirements of hazardous substances are kept and there is prevention against any possibility of contamination on any product or part.
All activities are based on documented procedures and require all necessary records to ensure due diligence.
The activities related to product realization requirements can be optimized from development of a risk analysis based on the aspects and impacts of each component or subassembly and of each activity performed during the production process. This risk analysis can assist in the prioritization of activities and cost reduction. Methods of risk management can be found in ISO/ IEC 31010 (ISO/IEC, 2009).
Section 8, related to measurement, analysis and improvement defines that must be implemented documented efficient ways to ensure that planned and implemented methods be executed, in order to conduct monitoring for the control of hazardous substances and prevent contamination of parts and products.
Companies that are still not adequate to a HSMS will have to define an implementation strategy. The flowchart in Figure 1 complements the text presented in the ABNT IECQ QC 080000 (ABNT, 2010) and summarizes the main steps to be taken toward compliance.
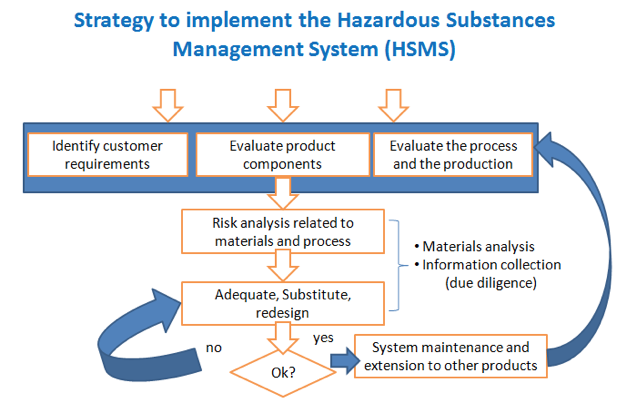
Figure 1. Implementation strategy to obtain warranty of products free of hazardous substances
Source: EWALD, 2011.
HSMS implementation decision may appear at the first moment extremely laborious and/or expensive, but if considered the structuring and prioritizing activities that are part of the implementation and the consistency and control of activities that the HSMS provides, these apparent costs may become in future new business opportunities by demonstrating due diligence to current and future customers, as well as, reducing the risks of non-compliant products put on the market.
The implementation strategy must be coherent and consistent with the size of the organization and always must be aligned with the management systems in place in the organization.
HSMS implementation in the Brazilian market had acceptance and companies interested in maintain CE (Conformité Européenne) marking are working to make its products and processes suitable.
The CE marking is a product conformity in applying guidelines of the European community, allowing producers and exporters to sell their products without restriction anywhere in the European market and indicates compliance with basic safety requirements under regulations. That differ according to the product, especially for the segments that were regulated by the revision of RoHS Directive (DIRECTIVE 2011/65/EU, 2011), occurred in 2011.
One of the segments affected by the revision of RoHS Directive is the Brazilian electromedical sector, where in an initial assessment of the segment, performed in 2009, considering medium-sized companies, it can be affirmed that these companies have the following characteristics: 1. suppliers to the governmental area; 2. exporters; 3. have high technology capabilities knowledge of their products, but don´t have information about the presence of hazardous substances in their products and are unaware about the status of their suppliers regarding this requirement or do not have objective evidence if the suppliers sells free of hazardous substances products or parts.
This sector has a strong regulation by ANVISA (Brazilian National Agency for Sanitary Vigilance) and all companies have implemented management systems, e.g. ISO 9001 (ABNT, 2008), ABNT NBR ISO 13485 (ABNT, 2004), Brazilian Resolution - RDC Nº 59 (ANVISA, 2000).
During implementation of a management system in this scenario, preliminary diagnosis made through interviews in nine companies of the Brazilian electromedical sector affected by the revision of RoHS Directive (DIRECTIVE 2011/65/EU, 2011) was prepared. These interviews aimed to assess the degree of understanding and meeting the requirements related to the HSMS and identify needs and opportunities for the implementation of a HSMS.
The preliminary diagnosis is detailed in Table 2. The analysis of the data demonstrates: necessity of training the electromedical market in requirements for environmentally sound management and ecodesign techniques; the need to create knowledge and experience about the occurrence of hazardous substances in products; the need to make product adaptation; the need to develop the supply chain (e.g. suppliers, subcontractors) and the sector clients.
Issues addressed on the degree of understanding and meeting requirements related to HSMS in electromedical sector and diagnostic |
|
Knowledge about the European Directives |
a. 7 of the companies interviewed already know RoHS and WEEE Directives or had some contact with this requirement. b. All of the companies are unaware of the standard QC 080000:2005. |
Use of hazardous substances |
Companies unaware the occurrence of hazardous substances that may be present in the product. |
Product recovery at the end of life |
a. 5 of the companies provide information to clients about procedures for disposal of products. b. 2 of the companies have a defined process for recycling or recovery of materials. |
Applying the concept of ecodesign in product |
No actions were identified regarding ecodesign |
Table 2 - Results of structured interviews and site visits in response to an invitation
by 09 Brazilian electromedical companies where the analysis was focused on a default
product chosen by the company interviewed
This table shows that for the Brazilian electromedical sector, there is a strong need for actions related to training and awareness of the various participants in the supply chain, including build the capacity of the laboratories that verify and certify the compliance of inputs and Brazilian electromedical products.
A revision of the standard IECQ QC 080000 (IECQ QC 080000, 2012) was approved in 2012 due to the maturity of the document and the diversity of regulations related to hazardous substances in electronic products in many countries.
As shown in Figure 2, there is a clear increase in the number of certificates issued for this management system, in a manner that the adequacy of the document becomes important after a maturation phase in the use of HSMS.
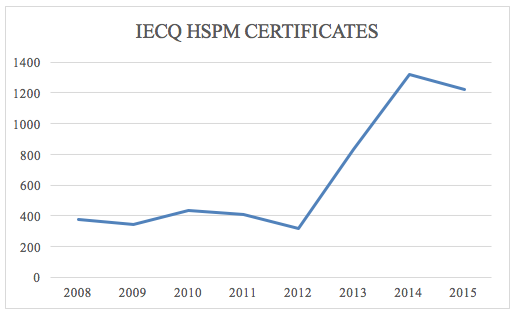
Figure 2- Annual emission of IECQ QC 080000 certificates
Source: IECQ System, 2016
Below we highlight the main changes in the new version of the document that let the application more extensive in relation to the list of hazardous substances to be controlled:
To accomplish the demands of an increasingly restrictive market and maintain its competitiveness, organizations must adopt a proactive approach adapting their products and process, especially to fulfill the European Directives, which are basis for other international markets.
Applying the requirements of the specification, ABNT IECQ/QC 080000 (ABNT, 2010) demonstrates an environmentally responsible attitude by the company, a fact that can potentially contribute to the expansion of the organization's business and contribute to minimize environmental impacts in their production activities.
A management system based on the requirements of ABNT IECQ/QC 080000 (ABNT, 2010) can provide the satisfaction and loyalty of customers by meeting their expectations and needs; reduce risks of non-compliance with laws and other regulations; strengthen the brand and provide market differentiation by introducing innovative products. Moreover, the system encourages actions in all stages of product life cycle, especially actions related to suppliers.
However, significant problems was found, especially related to lack of technical knowledge about the presence of hazardous substances in the supply chain, necessary information to meet the requirements of restriction of hazardous substances, mainly considering Brazilian producers of inputs, since there is no specific regulation in Brazil on this issue.
At this point, the conclusion is that the main opportunities is related to the qualification of the Brazilian market for the requirements to be satisfied and the technical knowledge related to the presence or lack of hazardous substances.
This qualification, however, demand efforts related to development of suitable materials or ability to adapt existing processes; implementation of concepts related to ecodesign, increasing of offer of accredited tests laboratories, development of test methods and standards in combination with international organizations.
ABNT NBR ISO 13485:2004 (2004). Produtos para saúde - Sistemas de gestão da qualidade - Requisitos para fins regulamentares. Rio de Janeiro: Associação Brasileira de Normas Técnicas.
ABNT NBR ISO 9001:2008 (2008). Sistema de gestão da qualidade - Requisitos. Rio de Janeiro: Associação Brasileira de Normas Técnicas.
ABNT IECQ/QC 080000:2010 (2010) Sistema de gestão para substâncias perigosas em produtos e componentes elétricos e eletrônicos – Requisitos. Rio de Janeiro: Associação Brasileira de Normas Técnicas.
ANVISA (2000). Resolução - RDC nº 59, de 27 de junho de 2000, Boas práticas de fabricação em saúde.
BRASIL (2010a) Instrução Normativa Nº 01, de 19 de janeiro de 2010, Ministério do Planejamento, Orçamento e Gestão.
BRASIL (2010b) Lei Nº 12.305, de 2 de agosto de 2010. Institui a Política Nacional de Resíduos Sólidos; altera a Lei no 9.605, de 12 de fevereiro de 1998; e dá outras providências.
BRASIL (2010 c) Decreto Nº 7404, de 23 de dezembro de 2010. Regulamenta a Lei no 12.305, de 2 de agosto de 2010, que institui a Política Nacional de Resíduos Sólidos, cria o Comitê Interministerial da Política Nacional de Resíduos Sólidos e o Comitê Orientador para a Implantação dos Sistemas de Logística Reversa, e dá outras providências.
DIRECTIVE 2002/96/EC (2003) of the European Parliament and of the council of 27 January 2003 on waste electrical and electronic equipment (WEEE), Official Journal of the European Union.
DIRECTIVE 2002/95/EC (2003) of the European Parliament and of the council of 27 January 2003 on the restriction of the use of certain hazardous substances in electrical and electronic equipment, Official Journal of the European Union.
DIRECTIVE 2011/65/EU (2011) of the European Parliament and of the council of 8 June 2011 on the restriction of the use of certain hazardous substances in electrical and electronic equipment (recast), Official Journal of the European Union.
DIRECTIVE 2012/19/EU (2012) of the European Parliament and of the council of 4 July 2012 on waste electrical and electronic equipment (WEEE) (recast), Official Journal of the European Union.
EWALD, M.R. (2011) Implantação dos requisitos da ABNT IECQ QC 080000. Seminário Interno e Treinamentos, 24 de agosto de 2011, Centro de Tecnologia da Informação Renato Archer.
IECQ QC 080000 (2005) Electrical and Electronic Components and Products - Hazardous Substance Process Management System Requirements (HSPM).
IECQ QC 080000 Electrical and Electronic Components and Products - Hazardous Substance Process Management System Requirements (HSPM), 3rd edition, 2012.
IECQ System (2016). Annual emission of IECQ QC 080000 certificates. Available from: http://certificates.iecq.org/iecq/iecqweb.nsf/(Export)?OpenAgent. Accessed on: 18 April 2016.
ISO/IEC 31010 (2009). Risk management -- Risk assessment techniques, 2009.
REGULATION (EC) Nº 1907 (2006) of the European Parliament and of the council of 18 December 2006 concerning the Registration, Evaluation, Authorization and Restriction of Chemicals (REACH), Official Journal of the European Union, 2006.
ROCHA, G.H.T. et. al. (2009). Diagnóstico da Geração de Resíduos Eletroeletrônicos no Estado de Minas Gerais. Minas Gerais: Fundação Estadual do Meio Ambiente – FEAM.
UK (2014) Department for Business, Innovation and Skills: Restriction of hazardous substances (RoHS) regulations 2012, Government Guidance Notes for RoHS.
1. Center for Information Technology Renato Archer, Campinas – SP, Brazil. Email: marcia.ewald@cti.gov.br
2. Federal Institute of Espirito Santo, Cariacica- ES, Brazil. Email: daniela.moraes@ifes.edu.br